Research Groups
Research Group "Aortic Lesions"
Diseases of the aorta are becoming increasingly common in an aging population and are of high relevance to society as a whole. Abdominal aortic aneurysms (AAA) and dissections (AD) are serious diseases of the aorta, the pathogenesis and therapy of which have not yet been adequately investigated.
The research group Aortic Lesions investigates the pathophysiology of these diseases. A special focus is on the investigation of pathomechanisms of established risk factors, such as age, hypertension and nicotine abuse. In addition to basic science questions, innovative therapies and diagnostic approaches are also pursued. A network of national and international scientists creates a stable foundation that enables us to share knowledge gained and to deepen our understanding of disease-specific processes. The overall goal of the research group is the translation of experimental findings into clinical practice.
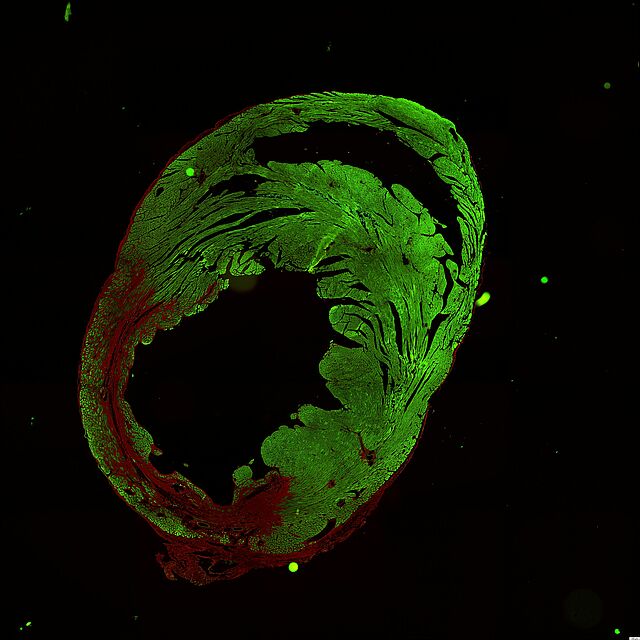
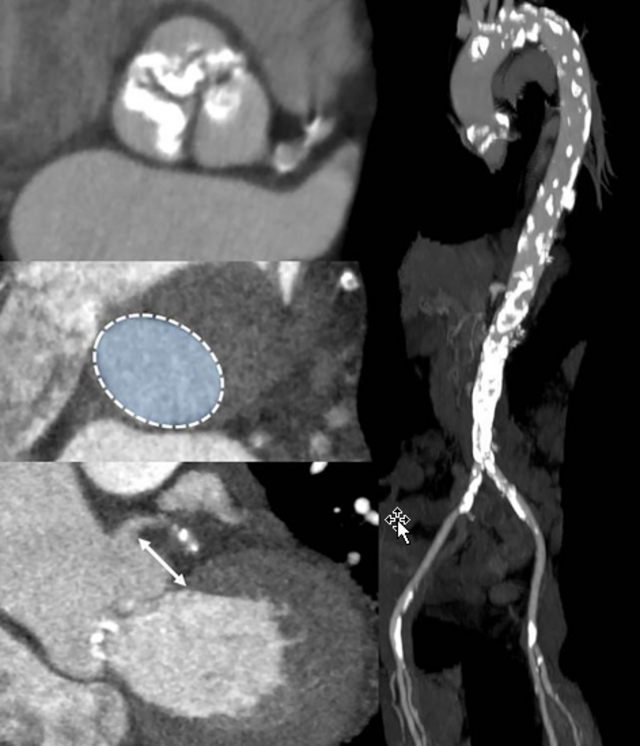
AAA is characterized by progressive destruction of the aortic wall. Elastin fragmentation by increased expression (top row right, red fluorescence) and activity (bottom row right, green-blue fluorescence) of matrix metalloproteinases (MMPs) mechanistically leads to an increase in aortic stiffness, which in turn is the contributing factor to the diameter progression of AAA. This process is also maintained by chronic inflammation, the genesis of which has not yet been fully elucidated. However, smoking conventional and e-cigarettes accelerates these processes.
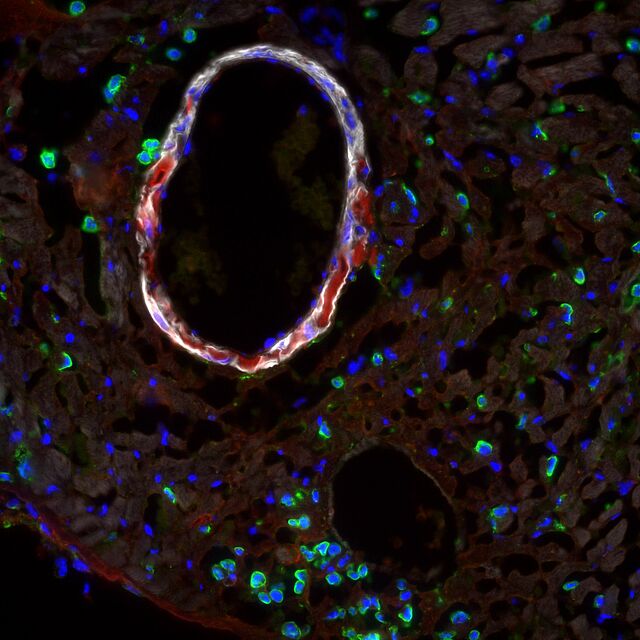
Most AAA harbor an intraluminal thrombus (ILT) that sub-totally lines the lumen. Such an ILT is present in up to 80% of an AAA. The role of the ILT remains the subject of current research approaches, and its role remains divergent. Whereas biomechanical studies suggest a rather protective effect on the overall stability of the AAA, molecular biological approaches described a destabilizing destructive effect on the AAA wall. Platelet activation is an obligatory prerequisite for ILT establishment and growth. This platelet activation at the ILT of an AAA appears to be of disease-specific interest.
AD represents a medical emergency associated with a significant pain event for the affected individual. AD results from a tear of the innermost endothelial cell layer, leading to the formation of a second false lumen within the aortic wall. After the acute event, there is successive laceration of the aortic wall. Due to the loss of stability, there is a long-term risk of formation of a post-dissection aneurysm with potential rupture. Mechanistically, processes that increase the likelihood of the occurrence of the acute event should be identified. In this regard, both biochemical and mechanical individual-based morphological processes and conditions are of great scientific interest. Close collaboration with researchers from the "bioenginnering" field is essential for the discussion of corresponding questions and hypotheses. Most recently, the role of small side branches called lumbar arteries, which arise from the false lumen of the AD, was shown to be underestimated. They seem to maintain patency of the false lumen. This could generate new therapeutic approaches in the future, as occlusion of these side branches may have therapeutic potential.
Our research group works on clinical projects as well as in the field of basic research on the following main topics:
Focus on Abdominal Aortic Aneurysm
- Exosomes as a novel therapeutic target in Aortic Aneurysm
- Effects of plasma therapy on Abdominal Aortic Aneurysm development
Focus on Acute Aortic Syndrome
- Disruption of the Endothelial Barier as a percusor for Aortic Dissection
- Aortic Arch Morphology and Geometry in acute aortic syndrom
Research Group "Cardiovascular Immune Biology"
- The immune system in cardiovascular biology
- Adaptive immunity, T cells and their subsets in atherosclerosis and myocardial infarction
- Function of co-stimulatory molecules in cardiovascular diseases and other inflammatory conditions
- Platelets as participants of immune mechanisms
- Role of T cells in ischemia/reperfusion injury
- Function of co-stimulatory molecules (e.g. CD40/CD40L, CD27/CD70) in acute myocardial infarction
- Cell type-specific role of CD40/CD40L in atherosclerosis
- Stroke-induced cardiovascular dysfunction
- Reiche, M. E., den Toom, M., Willemsen, L., van Os, B., Gijbels, M., Gerdes, N., Aarts, S., & Lutgens, E. (2019). Deficiency of T cell CD40L has minor beneficial effects on obesity-induced metabolic dysfunction.BMJ open diabetes research & care, 7(1), e000829. doi.org/10.1136/bmjdrc-2019-000829
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6936585/
-
Bianchini M, Duchêne J, Santovito D, Schloss MJ, Evrard M, Winkels H, Aslani M, Mohanta SK, Horckmans M, Blanchet X, Lacy M, von Hundelshausen P, Atzler D, Habenicht A, Gerdes N, Pelisek J, Ng LG, Steffens S, Weber C, Megens RTA. PD-L1expression on nonclassical monocytes reveals their origin and immunoregulatory function. Sci Immunol. 2019;4(36):eaar3054. doi:10.1126/sciimmunol.aar3054
https://immunology.sciencemag.org/content/4/36/eaar3054
- Winkels H, Meiler S, Lievens D, Engel D, Spitz C, Burger C, Beckers L, Dandl A, Reim S, Ahmadsei M, Hartwig H, Holdt LM, Hristov M, Megens RT, Schmitt M, Biessen EA, Borst J, Faussner A, Weber C, Lutgens E, Gerdes N. CD27 co-stimulation increases the abundance of regulatory t cells and reduces atherosclerosis in hyperlipidemic mice. Eur Heart J. 2017, 38(48):3590-3599. (PMID: 29045618)
https://academic.oup.com/eurheartj/article/38/48/3590/4554905
- Winkels H, Meiler S, Smeets E, Lievens D, Engel D, Spitz C, Bürger C, Rinne P, Beckers L, Dandl A, Reim S, Ahmadsei M, Van den Bossche J, Holdt LM, Megens RT, Schmitt M, de Winther M, Biessen EA, Borst J, Faussner A, Weber C, Lutgens E, Gerdes N. CD70 limits atherosclerosis and promotes macrophage function. Thromb Haemost. 2017, 117(1):164-175. (PMID: 27786334)
https://www.thieme-connect.de/products/ejournals/abstract/10.1160/TH16-04-0318
- Gerdes N, Seijkens T, Lievens D, Kuijpers MJ, Winkels H, Projahn D, Hartwig H, Beckers L, Megens RT, Boon L, Noelle RJ, Soehnlein O, Heemskerk JW, Weber C, Lutgens E. PlateletCD40 Exacerbates Atherosclerosis by Transcellular Activation of Endothelial Cells and Leukocytes. Arterioscler Thromb Vasc Biol. 2016, 36(3):482-90. (PMID: 26821950)
https://www.ncbi.nlm.nih.gov/pubmed/26821950
- Wang J, Sun C, Gerdes N, Liu C, Liao M, Liu J, Shi MA, He A, Zhou Y, Sukhova GK, Chen H, Cheng M, Kuzuya M, Murohara T, Zhang J, Cheng X, Jiang M, Shull GE, Rogers S, Yang CL, Ke Q, Jelen S, Bindels R, Ellison DH, Jarolim P, Libby P, Shi GP. Interleukin 18 function in atherosclerosis requires both the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med. 2015, 21(7):820-6. (PMID: 26099046)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4554539/
- Strodthoff D, Lundberg AM, Agardh HE, Ketelhuth DF, Paulsson-Berne G, Arner P, Hansson GK, Gerdes N. Lack of Invariant Natural Killer T Cells Affects Lipid Metabolism in Adipose Tissue of Diet-Induced Obese Mice. Arterioscler Thromb Vasc Biol. 2013, 33(6):1189-96. (PMID: 23520162)
https://www.ahajournals.org/doi/10.1161/ATVBAHA.112.301105
- Klingenberg R*, Gerdes N*, Badeau RM, Gisterå A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Lüscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013, 123(3):1323-34. (PMID: 23426179) (* indicates equal contribution)
https://www.jci.org/articles/view/63891
- Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, Hansson GK, Li N. Platelets regulate CD4⁺ T-cell differentiation via multiple chemokines in humans. Thromb Haemost. 2011, 106(2):353-62. (PMID: 21655676)
https://www.ncbi.nlm.nih.gov/pubmed/21655676
- Zirlik A, Maier C, Gerdes N, MacFarlane LA, Soosairajah J, Bavendiek U, Arens I, Ernst S, Bassler N, Missiou A, Patko Z, Aikawa M, Schönbeck U, Bode C, Libby P, Peter K. CD40L mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007, 115(12):1571-1580. (PMID: 17372166)
https://www.ncbi.nlm.nih.gov/pubmed/17372166
- Schönbeck U, Gerdes N, Varo N, Reynolds RS, Horton DB, Bavendiek U, Robbie LA, Ganz P, Kinlay S, Libby P. Oxidized low-density lipoprotein augments and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors limit CD40 and CD40L expression in human vascular cells. Circulation 2002, 106(23):2888-2893. (PMID: 12460867)
https://www.ncbi.nlm.nih.gov/pubmed/12460867
- Gerdes N, Sukhova GK, Libby P, Young JL, Reynolds RS, Schönbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002, 195(2):245-257. (PMID: 11805151)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2193607/
Research Group Leader | Director Cardiovascular Research Laboratory
Visit the official UKD website of Professor Gerdes for further information
Research Group "Cardiovascular Degeneration"
Research Group Leader
Prof. Judith (Jojo) Haendeler has been tenured after holding a DFG-funded Heisenberg professorship from 2014 to 2019. She is workhriebening since many years on signaling mechanisms, redox homeostasis and mitochondrial functions in cardiovascular aging and disease. Her work on Thioredoxin-1 showed that this small protein - besides serving as an oxidoreductase - has multiple additional functions, which are mediated through interactions with a plethora of cytosolic and nuclear proteins. Moreover, she was among the first to describe mitochondrial functions of Telomerase Reverse Transcriptase and demonstrated an essential role for it in the cardiovascular system in vivo, which she also showed for mitochondrial CDKN1B/p27. She is currently addressing the question of how to increase the mitochondrial levels of these two proteins to evaluate potential therapeutic options of such interventions. Prof. Haendeler is not only well embedded in the cardiovascular research activities in Düsseldorf, but she also fosters numerous national and international cooperations.
Research Group Leader
The focus of Prof. Joachim (Yogi) Altschmieds work is on molecular mechanisms regulating vascular and heart functions with a focus on transcriptional regulation. In that respect, he has described extra-nuclear functions of the transcription factor Grainyhead-like 3, which has protective functions in the endothelium. In addition, he is an expert in mouse genetics and has generated several unique new mouse and cell models to study organelle-specific functions of proteins, which are localized in the cell nucleus as well as in the mitochondria. Using these models, he is identifying and characterizing mitochondrial interaction partners of Telomerase Reverse Transcriptase and CDKN1B/p27 in close collaboration with Prof. Haendeler and partners at the Fritz Lipman Institute in Jena and other institutions in Germany and worldwide.
Research Group "Molecular Imaging"
In the past 10-15 years, it has become clear that inflammatory processes play an important role in the development and progression of multiple diseases, such as atherosclerosis, myocardial infarction but also cancer, or even neurological diseases. Noninvasive molecular imaging of inflammatory processes on a systemic and local level can help to gain a better understanding of the pathophysiological mechanisms and could identify novel therapeutic strategies.
One technology that enables the visualisation of inflammatory processes is combined 1H/19F magnetic resonance imaging (1H/19F MRI). For imaging of inflammation by 19F MRI, most often perfluorocarbon nanoemulsions (PFCs) are used that are avidly taken up by phagocytic immune cells such as monocytes/macrophages after intravenous injection. The accumulation and the anatomical location of these PFC-labeled cells within inflamed lesions can be unequivocally detected by 1H/19F MRI1–4. Of note, the hallmarks of 19F MRI are, on the one hand, that the NMR sensitivity is close to 1H and that 19F atoms are nearly absent from biological tissue. Accumulation of 19F atoms can, therefore, be detected with high sensitivity and specificity.
To expand the repertoire of the structures and cells that can be visualized by 1H/19F MRI, we built up a platform for the active targeting of PFCs5. To this end, we utilised the conjugation of specific targeting-ligands in combination with the surface PEGylation for specific visualization of thrombi6, activated platelets7, specific progenitor cells8 or neutrophils9. More recently, we were also able to simultaneously visualize thromboinflammatory lesions by targeting monocytes/macrophages, active factor XIII, and fibrin using PFCs with individual spectral signatures10.

Figure 1: Perfluorocarbons can be targeted to different cells and structures involved in the development and progression of inflammatory or thromboinflammatory processes that can be visualised using specific targeting ligands and perfluorocarbons with individual spectral identities. The multicolor 19F image (upper left) shows the simultaneous detection of inflammatory lesions (red = monocytes/macrophages) and thrombus formation (FXIIIa-activity = cyan; fibrin = magenta). This figure is derived and adapted from Kleimann et al. 202311. (Parts of this figure were taken from Flögel et al. 202110, and schematic drawings were obtained from Smart.Servier.com.
Currently, our group is working further optimising the PFCs to enhance sensitivity, specificity, and the expansion of the applications to investigate the relevance of different types of priming the immune system for the development and progression of cardiovascular diseases.
For further information about the technological background and other MRI applications, please visit the website of the Department for Experimental Cardiovascular Imaging.
- Flögel, U. et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 118, 140–148 (2008).
- Ebner, B. et al. Early Assessment of Pulmonary Inflammation by 19F MRI In Vivo. Circ. Cardiovasc. Imaging 3, 202–210 (2010).
- Flögel, U. et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 4, 146ra108 (2012).
- Temme, S. et al. Beyond Vessel Diameters: Non-invasive Monitoring of Flow Patterns and Immune Cell Recruitment in Murine Abdominal Aortic Disorders by Multiparametric MRI. Front. Cardiovasc. Med. 8, 1421 (2021).
- Temme, S., Grapentin, C., Güden-Silber, T. & Flögel, U. Active Targeting of Perfluorocarbon Nanoemulsions. in Fluorine Magnetic Resonance Imaging 97–133 (CRC Press, 2016).
- Temme, S. et al. Noninvasive Imaging of Early Venous Thrombosis by 19F Magnetic Resonance Imaging With Targeted Perfluorocarbon Nanoemulsions. 131, (2015).
- Wang, X. et al. Fluorine-19 Magnetic Resonance Imaging of Activated Platelets. J. Am. Heart Assoc. 9, e016971 (2020).
- Straub, T. et al. MRI-based molecular imaging of epicardium-derived stromal cells (EpiSC) by peptide-mediated active targeting. Sci. Rep. 10, 21669 (2020).
- Bouvain, P. et al. Non-invasive mapping of systemic neutrophil dynamics upon cardiovascular injury. Nat. Cardiovasc. Res. (2023) doi:10.1038/s44161-022-00210-w.
- Flögel, U. et al. Multi-targeted 1H/19F MRI unmasks specific danger patterns for emerging cardiovascular disorders. Nat. Commun. 12, 5847 (2021).
- Kleimann, P. et al. Bildgebung von thromboentzündlichen Prozessen durch multispektrale 19F-MRT. Gefässchirurgie (2023) doi:10.1007/s00772-023-01045-w.





![[Translate to English:] 3D-Darstellung der CD45-positiven lebenden Immunzellen und ihrer Diversität im murinen Herzen an Tag 5 nach einem Myokardinfarkt.](/fileadmin/_processed_/5/9/csm_Macorphage_UMAP_53ff18f17f.jpg)