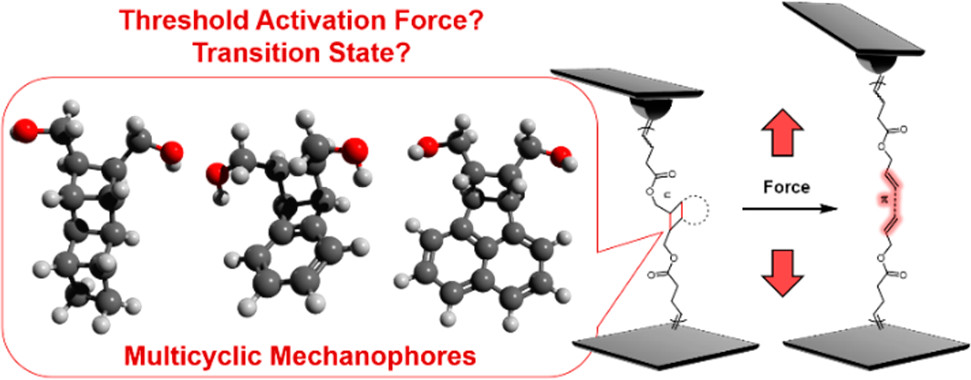
We have recently reported a series of ladder-type cyclobutane mechanophores, polymers of which can transform from nonconjugated structures to conjugated structures and change many properties at once. These multicyclic mechanophores, namely, exo-ladderane/ene, endo-benzoladderene, and exo-bicyclohexene-peri-naphthalene, have different ring structures fused to the first cyclobutane, significantly different free energy changes for ring-opening, and different stereochemistry. To better understand their mechanochemistry, we used single molecule force spectroscopy (SMFS) to characterize their force–extension behavior and measure the threshold forces. The threshold forces correlate with the activation energy of the first bond, but not with the strain of the fused rings distal to the polymer main chain, suggesting that the activation of these ladder-type mechanophores occurs with similar early transition states, which is supported by force-modified potential energy surface calculations. We further determined the stereochemistry of the mechanically generated dienes and observed significant and variable contour length elongation for these mechanophores both experimentally and computationally. The fundamental understanding of ladder-type mechanophores will facilitate future design of multicyclic mechanophores with amplified force-response and their applications as mechanically responsive materials.
Read more in:
Understanding the Mechanochemistry of Ladder-Type Cyclobutane Mechanophores by Single Molecule Force Spectroscopy
Matías Horst, Jinghui Yang, Jan Meisner, Tatiana B. Kouznetsova, Todd J. Martínez, Stephen L. Craig, Yan Xia
J. Am. Chem. Soc., 143, (31), 12328–12334, 2021
https://doi.org/10.1021/jacs.1c05857